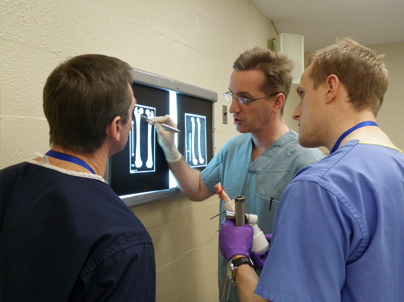
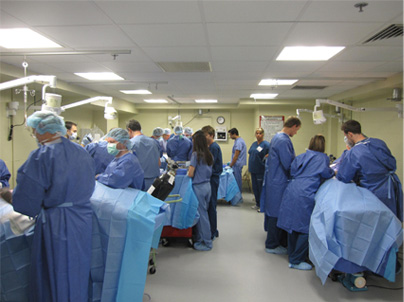
WORKSHOPS
BioMedtrix conducts product workshops throughout the United States and Europe. Each workshop details the application and surgical technique for a specific product such as the Universal Hip (BFX® and CFX® systems), Small Breeds Hip, TATE Elbow®, or I-Loc® Nail. Workshops consist of classroom instruction supported by lab experience, offering participants the opportunity to use the surgical instrumentation in order to familiarize themselves with the procedure.
OF US VETERINARY UNIVERSITIES REPRESENT BIOMEDTRIX PROCEDURES
PROCEDURES & WORKSHOPS ARE PIONEERED BY LEADERS IN VETERINARY ORTHOPEDICS
REGISTRY OF APPROVED CONTINUING EDUCATION (RACE) APPROVED WORKSHOPS
UPCOMING WORKSHOPS
Product Mentorship
Surgeons who have completed a BioMedtrix workshop may choose to participate in the Product Mentorship Program. This program incurs additional costs and is designed to support new total joint replacement surgeons as they progress beyond attendance at a total joint replacement workshop, towards performing clinical total joint replacement procedures. Surgeons who complete this program will have demonstrated an understanding of the concepts necessary for successful clinical results.
The program offers three potential pathways to practice the procedure, gain confidence, and validate a surgeon’s comprehension of the concepts required for successful clinical results. One of the following three pathways may be selected: Cadaver Implantation, Surgeon Assist Training, or Mentor Training.
Completion of the Mentorship Program is a required process prior to purchasing implants from BioMedtrix for total joint systems. The Product Mentorship Program is offered for the Universal Hip and Small Breeds Hip systems.
RACE APPROVED
BioMedtrix Workshops for the Universal Hip, TATE Elbow, and I-Loc Nail have been approved by RACE (Registry of Approved Continuing Education), a national clearinghouse for endorsement of veterinary continuing education providers and their programs.
Programs approved by RACE are acknowledged by most states and several provinces in Canada. The organization is a service of the American Association of Veterinary State Boards.
RACE approval allows veterinarians from around the country to continue their education and thereby maintain their state licensing requirements.